Abstract
Introduction
Currently, there are three FDA-approved novel multiple myeloma (MM) oral agents commonly used in clinical practice in the United States: two immunomodulators (lenalidomide [len] and pomalidomide [pom]) and one proteasome inhibitor (ixazomib [ixa]). The financial burden faced by patients with MM on these drugs can be considerable due to increasing long-term survival and extended periods of continuous single agent maintenance or multidrug combination regimens. To better understand the financial burden associated with extended use of these medications, we sought to leverage a real-world, all-claims insurance database to examine the outpatient drug copayments, associated fill patterns, and duration of therapy of these MM oral agents, stratified by insurance type. We hypothesize that copayments associated with oral MM agents will comprise a major portion of the overall drug copayment per patient, and fill patterns and duration of therapy of these medications will vary across patients of different insurance types.
Methods
We utilized outpatient drug claims derived from the 2014-2018 IBM/Truven MarketScan Commercial, Medicare Advantage, and Medicaid all-claims databases and extracted all outpatient claims from patients who filled at least one prescription (≥28-day duration) for len, pom, or ixa. Annual outpatient medication copayment and number of outpatient scripts were calculated for each patient, both for oral MM agents only and all outpatient prescriptions. Copayments were normalized to the cost per annum. Multivariable linear regression was used to compare outcomes between patients of the three insurance types.
Results
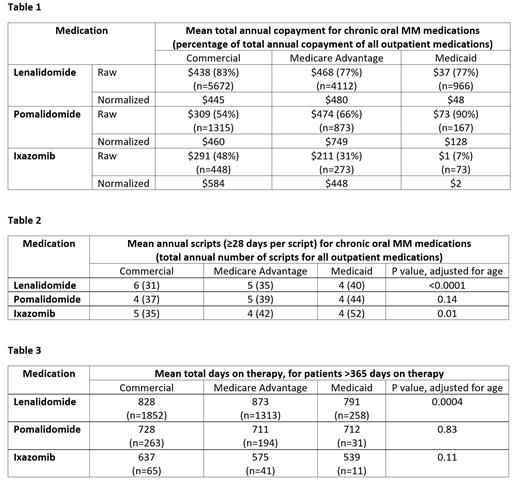
From 2014-2018, we identified 10,750 patients on len, 2,355 patients on pom, and 794 patients on ixa. These included patients with commercial insurance (53.5%), Medicare Advantage (37.8%), and Medicaid (8.7%). The average adjusted annual copayment per patient of len was $445 for commercial, $480 for Medicare Advantage, and $48 for Medicaid (Table 1). Len copayment comprised of 77-83% of the total outpatient drug copayment per patient. For pom, adjusted copayments were $460 for commercial, $749 for Medicare Advantage, and $128 for Medicaid. Pom copayment comprised 54-90% of the total. For ixa, adjusted copayments were $584 for commercial, $448 for Medicare Advantage and $2 for Medicaid. Ixa copayment comprised 7-48% of the total.
Patients with commercial insurance generally filled more scripts for oral MM agents per year (6 len, 4 pom, 5 ixa) compared to those with Medicare Advantage (5 len, 5 pom, 4 ixa) and Medicaid (4 len, 4 pom, 4 ixa) (Table 2). However, patients with commercial insurance filled fewer overall outpatient scripts per year (31-37) compared to Medicare Advantage (35-42) and Medicaid (40-52). Insurance type was associated with the number of scripts filled for len (p<0.0001) and ixa (p=0.01) after adjustment for age, but not pom (p=0.14).
Among patients on oral MM therapy for more than one year, insurance type was associated with time on therapy for len (828 days for commercial, 873 days for Medicare Advantage, and 791 days for Medicaid, p=0.0004) (Table 3). However, insurance type was not associated with time on therapy for pom (p=0.83) or ixa (p=0.11).
Discussion
Our results demonstrate that copayments for oral MM agents comprise the majority of total outpatient drug copayments for patients with MM, suggesting that the out-of-pocket costs of these agents may be a key driver of financial burden. In addition to differences observed in the oral MM drug copayments, insurance type was also associated with the number of scripts filled and time on therapy for patients taking len. Thus, this suggests insurance type is also linked to drug utilization patterns and may indicate a differential financial burden in patients with MM on chronic oral therapy.
Reducing the cumulative impact of financial toxicity for patients with MM is an important consideration for prescribers, payors, and health systems to achieve optimal clinical outcomes. Although our current dataset lacks diagnostic and treatment data, further correlative studies incorporating care utilization data, including inpatient admissions and outpatient clinical visits, are in progress.
Pianko: Karyopharm: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal